
Chinese Medical Journal article unveils metabolic strategies to enhance CAR-T cell therapy
While cancer remains one of the leading global causes of death, advancements in cancer therapies have significantly improved its manageability and potential for cure. Among these revolutionary cancer treatments are chimeric antigen receptor-T (CAR-T) cells, genetically engineered to combat cancer. Despite demonstrating clinical efficacy in treating hematologic tumors, CAR-T cell therapy still faces limitations in treating solid tumors. Furthermore, metabolic obstructions within the tumor microenvironment (TME) present additional challenges in fully realizing the therapeutic potential of this treatment approach.
Credit: Chinese Medical Journal
While cancer remains one of the leading global causes of death, advancements in cancer therapies have significantly improved its manageability and potential for cure. Among these revolutionary cancer treatments are chimeric antigen receptor-T (CAR-T) cells, genetically engineered to combat cancer. Despite demonstrating clinical efficacy in treating hematologic tumors, CAR-T cell therapy still faces limitations in treating solid tumors. Furthermore, metabolic obstructions within the tumor microenvironment (TME) present additional challenges in fully realizing the therapeutic potential of this treatment approach.
Growing evidence suggests that competition with tumor cells for metabolic resources and changes in metabolic processes surrounding tumors significantly disrupt CAR-T cell therapy. However, enhancing T cell metabolic fitness through supplementary drugs and therapies has shown to notably boost their vitality and anti-tumor activity. Consequently, there is a surge in clinical research for developing innovative strategies to improve the efficacy of CAR-T cells by targeting metabolic processes.
In this context, a research team led by Dr. Yi Zhang from the First Affiliated Hospital of Zhengzhou University, China, conducted an extensive systematic review to examine how different metabolites reprogram T cells in the TME and explore metabolic interventions that could restore or enhance the efficacy of CAR-T cell therapy, based on recent studies. “The main aim of this review is to offer new perspectives for overcoming obstacles in the tumor microenvironment and enhance CAR-T cells’ therapeutic efficacy, particularly in treating solid tumors,” says Dr. Zhang. The article has been published online in the Chinese Medical Journal on March 19, 2024.
According to the article, T cells typically activate in response to foreign antigens, like tumors, leading to rapid proliferation and immune response stimulation. To sustain their activation, proliferation, and anti-tumor activity, T cells rely on normal metabolic processes with optimum levels of metabolites including glucose, fatty acids, and amino acids, among others, for nutrients and energy. However, in the TME these metabolites are also consumed by tumor cells to support their growth, limiting their availability and uptake by T cells.
Furthermore, the interaction of tumor cells with these metabolites often leads to abnormal metabolic conditions in the TME, making it inhospitable for T cells to grow, survive, metabolize, and exert immune function. For instance, glycolytic metabolism of cancer cells generates large amounts of lactate, making the TME more acidic and thus inhibiting T cell functions. Similarly, high accumulation of lipids in the TME, particularly ultra-long-chain fatty acids or cholesterol, impairs the tumor-infiltrating capability of T cells and other lymphocytes. Other abnormal metabolic changes can induce increased interstitial fluid pressure, reduced oxygen availability, and mitochondrial dysfunction within cells.
To address these challenges, scientists are continuously striving to develop practical and effective metabolic interventions capable of enhancing the metabolic adaptability and anti-tumor capabilities of CAR-T cells. Based on recent evidence in this field, the review article provides a synopsis of key strategies that have shown promising results. These strategies are broadly classified into three major approaches.
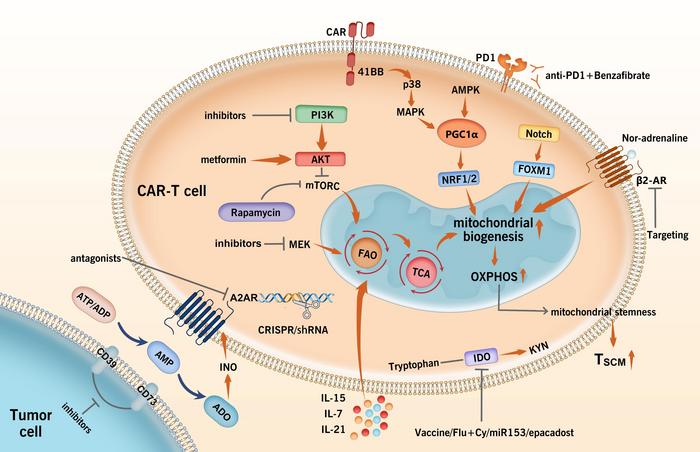
The first approach aims to mitigate the inhibitory effects of immunosuppressive metabolites in the TME by either reducing their activity or concentration or by enhancing CAR-T cell function. This can be achieved through gene editing or novel drug delivery systems using nanotechnology. For instance, genetic short hairpin RNA, CRISPR gene editing, or targeted pharmacological compounds can inhibit adenosine, an immunosuppresive metabolite in the TME, from binding with T-cells receptors, enabling proper T-cell activation and response, even against solid tumors.
The second intervention method aims to enhance metabolite uptake by T-cells. Different enzymes regulate the uptake and utilization of metabolites, but these are often insufficient within the hostile TME. To address this, CAR-T cells can be modified or reinforced with necessary enzymes like ornithine transcarbamylase or argininosuccinate synthase, which aid in synthesizing vital metabolites when their TME concentrations are low.
The third approach looks at improving mitochondrial metabolism to support CAR-T cell activity. By modifying specific mitochondrial proteins through genetic changes or targeted drug therapy, this approach boosts anti-tumor function by improving mitochondrial mass and biogenesis, leading to better differentiation, persistence, and tumor-killing ability, especially in solid tumors.
These metabolism-focused strategies underscore how modern technology can help overcome CAR-T cell treatment barriers to effectively treat both hematological and solid cancers. “By using innovative engineering techniques and combination therapies, we believe that CAR-T cells have immense potential to overcome various metabolic and physiological constraints to offer cancer patients a safe, long-term, and durable remission,” concludes Dr. Zhang.
***
Reference
DOI: https://doi.org/10.1097/CM9.0000000000003046
Journal
Chinese Medical Journal
DOI
10.1097/CM9.0000000000003046
Method of Research
Systematic review
Subject of Research
Cells
Article Title
Targeting metabolism to improve CAR-T cells therapeutic efficacy
Article Publication Date
19-Mar-2024
COI Statement
None