
Durvalumab and Tremelimumab before surgery in patients with HR+/HER2-negative breast cancer
“[…] this trial was stopped early after 3 of the first 8 enrolled patients experienced immunotherapy-related toxicity or suspected disease progression […]”
Credit: 2024 Garber et al.
“[…] this trial was stopped early after 3 of the first 8 enrolled patients experienced immunotherapy-related toxicity or suspected disease progression […]”
BUFFALO, NY- March 27, 2024 – A new research paper was published in Oncotarget’s Volume 15 on March 19, 2024, entitled, “Durvalumab and tremelimumab before surgery in patients with hormone receptor positive, HER2-negative stage II–III breast cancer.”
In this new study, researchers Haven R. Garber, Sreyashi Basu, Sonali Jindal, Zhong He, Khoi Chu, Akshara Singareeka Raghavendra, Clinton Yam, Lumarie Santiago, Beatriz E. Adrada, Padmanee Sharma, Elizabeth A. Mittendorf, and Jennifer K. Litton from the University of Texas MD Anderson Cancer Center, Brigham and Women’s Hospital, Dana-Farber Brigham Cancer Center, and Harvard Medical School conducted a clinical trial to assess the feasibility of enrolling patients with Stage II or III hormone receptor positive (HR+)/HER2-negative breast cancer to pre-operative dual PD-L1/CTLA-4 checkpoint inhibition administered prior to neoadjuvant chemotherapy (NACT).
“This feasibility study was conducted to begin testing the hypothesis that dual checkpoint blockade would increase TIL and enhance the response to subsequent NACT in patients with stage II or III HR+/HER2-negative breast cancer.”
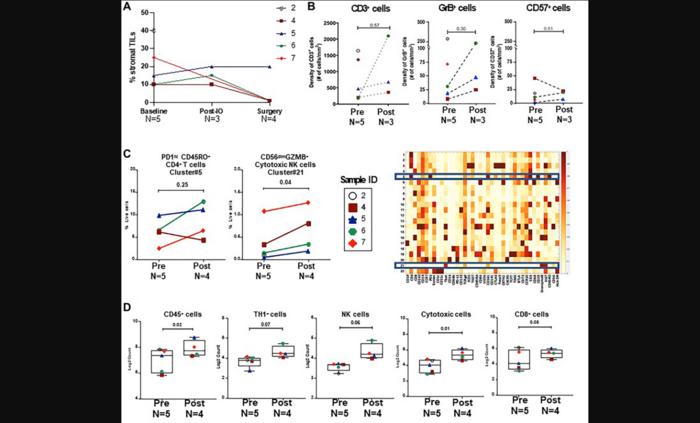
Eight eligible patients were treated with upfront durvalumab and tremelimumab for two cycles. Patients then received NACT prior to breast surgery. Seven patients had baseline and interval breast ultrasounds after combination immunotherapy and the responses were mixed: 3/7 patients experienced a ≥30% decrease in tumor volume, 3/7 a ≥30% increase, and 1 patient had stable disease. At the time of breast surgery, 1/8 patients had a pathologic complete response (pCR).
The trial was stopped early after 3 of 8 patients experienced immunotherapy-related toxicity or suspected disease progression that prompted discontinuation or a delay in the administration of NACT. Two patients experienced grade 3 immune-related adverse events (1 with colitis, 1 with endocrinopathy). Analysis of the tumor microenvironment after combination immunotherapy did not show a significant change in immune cell subsets from baseline.
“There was limited benefit for dual checkpoint blockade administered prior to NACT in our study of 8 patients with HR+/HER2-negative breast cancer.”
Continue reading: DOI: https://doi.org/10.18632/oncotarget.28567
Correspondence to: Haven R. Garber
Email: [email protected]
Keywords: breast cancer, ER positive, immunotherapy, neoadjuvant chemotherapy, tumor microenvironment
Click here to sign up for free Altmetric alerts about this article.
About Oncotarget: Oncotarget (a primarily oncology-focused, peer-reviewed, open access journal) aims to maximize research impact through insightful peer-review; eliminate borders between specialties by linking different fields of oncology, cancer research and biomedical sciences; and foster application of basic and clinical science.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
To learn more about Oncotarget, visit Oncotarget.com and connect with us on social media:
- X, formerly Twitter
- YouTube
- Spotify, and available wherever you listen to podcasts
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact [email protected].
Oncotarget Journal Office
6666 East Quaker Street., Suite 1A
Orchard Park, NY 14127
Phone: 1-800-922-0957 (option 2)
###
Journal
Oncotarget
DOI
10.18632/oncotarget.28567
Method of Research
Randomized controlled/clinical trial
Subject of Research
People
Article Title
Durvalumab and tremelimumab before surgery in patients with hormone receptor positive, HER2-negative stage II–III breast cancer
Article Publication Date
19-Mar-2024