Memories of mitosis: Molecular mechanism that detects defects during cell division could aid cancer treatment
Every day, our cells are hard at work multiplying. Cell division is a precise process, but sometimes this process is impaired and diseases like cancer occur. Mitosis is one of the most important phases in the cell cycle. During this phase a cell’s DNA is split into two equal sets of chromosomes and it divides into two genetically identical daughter cells.
Credit: OIST
Every day, our cells are hard at work multiplying. Cell division is a precise process, but sometimes this process is impaired and diseases like cancer occur. Mitosis is one of the most important phases in the cell cycle. During this phase a cell’s DNA is split into two equal sets of chromosomes and it divides into two genetically identical daughter cells.
Prof. Franz Meitinger, head of the Cell Proliferation and Gene Editing Unit at the Okinawa Institute of Science and Technology (OIST), Dr. Hazrat Belal, a researcher at the unit, and their collaborators at the University of California, San Diego, have found a molecular mechanism that prevents multiplication of potentially dangerous cells by measuring the duration of mitosis. They have shown in their paper published in Science, that this mechanism – the Mitotic Stopwatch Complex – gets stronger with time as cells divide, leading to the removal of abnormal cells to protect the organism.
The Mitotic Stopwatch Complex
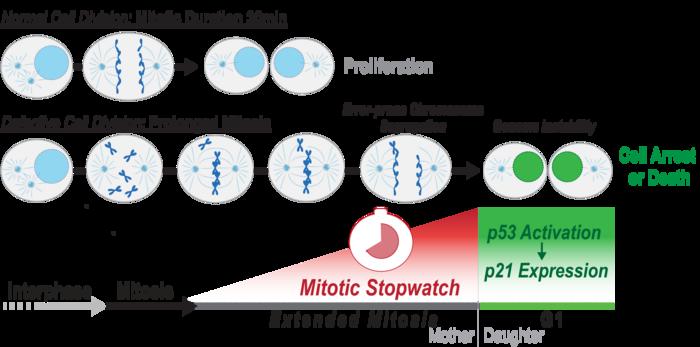
Normally, when a cell divides, it makes exact copies of its chromosomes – thread-like structures containing DNA which carries genetic information – and each of the new daughter cells receives a perfect copy. However, sometimes one cell might get too many chromosomes and the other might not get enough, a phenomenon known as ‘chromosome missegregation’.
Mitosis usually takes around 30 minutes to complete, but when cells have a defect, they need more time to organize the chromosomes and segregate them to the daughter cells. This delay results in what the researchers have called the Mitotic Stopwatch – a complex that forms when the cells experience unusual, prolonged mitosis.
“This complex doesn’t form during a normal mitosis, only when it takes longer. The defects in the cells are not directly recognized by the cells, but what the cells can measure is how long they spend in mitosis, and they use this information to understand how well mitosis happens,” Prof. Meitinger explained. “We wanted to understand how the molecular mechanism protects the organism from cancer development.”
The complex starts forming 30 minutes after mitosis starts and after the cell exits the extended mitosis it becomes active in the new daughter cells. This activation triggers other factors that can either permanently arrest or kill the cells.
“You have a signal that accumulates and when mitosis is long enough it can induce immediate cell arrest or cell death, but if you have a moderately prolonged mitosis, you have partial activation of this pathway, so the cells can still go on and divide, but if the cell has a moderately prolonged mitosis once again it will arrest,” Dr. Belal said.
Researchers knew there was a connection between prolonged mitosis and cell arrest but did not know how cells ‘sensed’ prolonged mitosis to cause cell arrest. This study clarifies how this works.
The Mitotic Stopwatch Complex consists of 3 proteins: p53 binding protein 1, USP28 and p53 protein itself. These proteins only interact during unusually longer mitosis (longer than 30 minutes). During this prolonged mitosis, more and more of the complex is formed. The more complex formed, the stronger the result – this could either be cell arrest or cell death, depending on the cell type.
p53 protein, known as a tumor suppressor, stops the growth of potentially damaged cells and prevents their proliferation. The scientists found that an enzyme, called PLK1 (a kinase), is responsible for triggering the formation of the complex. PLK1 is active during normal mitosis, but for unknown reasons only induces complex formation during prolonged mitosis.
When the complex is formed it can stabilize and activate the tumor suppressor p53 which can then act as a transcription factor (proteins that turn genes off and on, ensuring they are correctly expressed in the right cells at the right time). This discovery provides new insights into the role of these proteins during prolonged mitosis and their role in the removal of potentially dangerous cells that can cause cancer.
“When a little bit of the complex is made which is not enough to arrest the cells, the complex stays stable even in the granddaughter cells and accumulates. The granddaughter cells can remember the moderately prolonged state of mitosis in the grandmother cells,” Prof. Meitinger stated.
While every cell in our body has the potential for mitotic delay, it is less common in normal cells. A mitotic delay is more likely to happen in damaged cells, and the Mitotic Stopwatch is likely to act as a surveillance mechanism that removes these cells. In cancer cells there is often an even longer and flawed mitosis: in these cells this pathway is often inactive because it has mutated to have a flawed mitosis to drive cancer development.
Fate of cells
To monitor these cellular pathways, the researchers used live cell imaging which involved observing cells under a microscope over a three-day period. They temporarily extended the mitosis phase by introducing a mitotic inhibitor. After a few hours, the cells enter a state of prolonged mitosis, effectively ‘stuck’ in this phase. They then remove the inhibitor, allowing the cells to proceed with division.
Over the next three days, the cells were observed to determine their fate – whether they continue to divide, arrest, or die. This method allowed the researchers to show how cells detect prolonged mitosis and subsequently initiate cell arrest or death.
Dr. Belal noted that the most challenging part of the experiment was tracking the cells, as they move a lot and sometimes go out of frame under the microscope. Each experiment required the analysis of at least 160 individual cells, and many experiments had to be conducted to determine the mechanism of the Mitotic Stopwatch and its functionality across normal and cancer cell types. Every cell had to be analyzed individually. This meticulous process allowed the scientists to fully understand how the cells respond to extended mitosis in different situations.
The scientists used a technique called CRISPR-Cas9 to turn off certain genes and then studied the effects on the p53 protein. They found that some gene mutations can stop the protein from working properly. To understand this better, they are now studying the proteins that form the Mitotic Stopwatch Complex to see how they interact under different conditions.
The identification of the Mitotic Stopwatch has potential clinical applications. Some cancers maintain an active Mitotic Stopwatch, which makes them sensitive to anti-mitotic drugs that play an important role in cancer treatment by targeting cell division. These drugs are currently in clinical use or development. “If we could determine the activity of the mitotic stopwatch in individual cancers, we might be able to predict how these cancers respond to treatment with anti-mitotic drugs,” Prof. Meitinger stated. The researchers hope that their findings will eventually aid in the treatment of certain cancers.
Journal
Science
DOI
10.1126/science.add9528
Article Title
Control of cell proliferation by memories of mitosis
Article Publication Date
28-Mar-2024