The Lancet: Many people with breast cancer ‘systematically left behind’ due to inaction on inequities and hidden suffering
**Embargo: 23.30 [UK time] / 19.30 [ET], Monday 15 April 2024**
Credit: The Lancet
**Embargo: 23.30 [UK time] / 19.30 [ET], Monday 15 April 2024**
Peer-reviewed/Literature review, Survey, and Opinion/People
Embargoed access to the papers and contact details for authors and patient advocates are available in Notes to Editors at the end of the release.
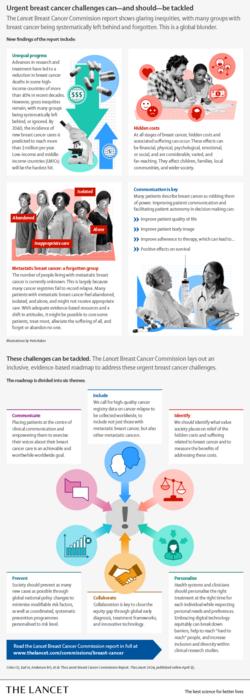
Breast cancer is now the world’s most common cancer; at the end of 2020, 7.8 million women were alive having been diagnosed in the previous five years. In the same year, 685,000 women died from the disease. Despite significant improvements in research, treatment, and survival, gross inequities persist, and many patients are being systematically left behind. This is a global blunder, says a new Lancet Commission.
An example of unequal progress in breast cancer relates to patients with metastatic breast cancer (MBC). The number of people living with MBC is unknown and many feel abandoned. Recording relapse in cancer registries to identify those with MBC and shifting negative societal attitudes towards MBC could facilitate optimal support for this patient population.
Hidden breast cancer costs and suffering can be financial, physical, psychological, emotional, and social, with impacts on patients, families, and wider society. Many of the costs associated with breast cancer are not adequately measured and remain overlooked by policymakers and society. New tools and metrics are required to expose these costs so that the needs of people affected by the disease can be met worldwide.
Women with breast cancer often report a sense of disempowerment after diagnosis. The Commission suggests better patient-health professional communication is a crucial intervention that can improve quality of life, body image, and adherence to therapy – with positive impacts on survival.
At the end of 2020, 7.8 million women were alive having been diagnosed with breast cancer in the previous five years; this reflects progress in research and cancer management that has led to a decrease of over 40% in breast cancer mortality in most high-income countries (HICs). However, 685,000 women died from the disease in 2020, and glaring inequities and suffering related to physical symptoms, emotional despair, and financial burden are often hidden and inadequately addressed. A new Lancet Commission sets out recommendations to tackle these urgent challenges in breast cancer.
Estimates suggest that global breast cancer incidence will rise from 2.3 million new cases in 2020 to over 3 million by 2040, and one million deaths from the disease per year are projected by 2040. Low- and middle-income countries are disproportionately affected.
Although breast cancer is the most common cancer, gaps in knowledge continue to prevent effective action. For example, the number of people living with metastatic breast cancer is not known, hampering the provision of treatment and care. At the same time, the scale of breast cancer-related suffering and other costs are not well-measured; “society and policymakers currently see only the tip of an iceberg”, say Commission authors.
The Commission’s lead author, Professor Charlotte Coles, Department of Oncology, University of Cambridge, UK, a National Institute for Health and Care Research (NIHR) Research Professor and Oncology Consultant at Cambridge University Hospitals NHS Foundation Trust, says: “Recent improvements in breast cancer survival represent a great success of modern medicine. However, we can’t ignore how many patients are being systematically left behind. Our Commission builds on previous evidence, presents new data, and integrates patient voices to shed light on a large unseen burden. We hope that, by highlighting these inequities and hidden costs and suffering in breast cancer, they can be better recognised and addressed by health care professionals and policymakers in partnership with patients and the public around the world.”
The number of people with MBC breast cancer is unknown
Even though 20-30% of patients with early breast cancer experience relapse, relapse is not typically recorded by most national cancer registries. Therefore, the number of patients living with MBC is not known. Meeting the needs of an under-measured patient population is difficult, and feelings of abandonment and isolation are common among those living with MBC as a result.
The Commission highlights how, in the last decade, MBC outcomes have improved considerably.
The median overall survival for two MBC subtypes (HER2-positive and ER-positive/HER2-negative), which include approximately 85% of MBC patients, has reached five years where recommended therapies are made available. Some patients may now live 10 years or longer with metastatic disease. In a Commission survey of 382 healthcare professionals (70% of whom were oncologists; more than half with a clinical focus on breast cancer), 55% agreed that specific subtypes of MBC may become curable, and 75% agreed that MBC will become a chronic disease (see Panel 24, page 21).
“MBC remains poorly understood by the public, policymakers, and even health care professionals”, says Lesley Stephen, collaborator and patient advocate. She continues: “Some patients have told me that they feel ‘written off’. This sense of being ignored and left behind can mean they are less likely to seek help or engage with research that could help them. A diagnosis of MBC should not stop a person’s contribution to society, but patients with metastatic disease need more support and information in order to feel valued.”
Authors make a case for a minimum of 70% of registries worldwide to record cancer stage and relapse. These data could drive significant improvements in MBC care, outcomes, and emotional wellbeing among patients. Initiatives that promote societal inclusion of people living with MBC are also paramount; for example, changes to labour market laws that empower more flexible working arrangements. With a shift in perception, it may be possible to treat most, alleviate suffering for all, and forget no-one living with MBC, the Commission argues.
Exposing the wide-ranging yet hidden costs of breast cancer
The associated costs of breast cancer—including physical, psychological, social, and financial costs—are immense but under-recognised. Indeed, many of these costs are not captured by current global health metrics.
In response, the Commission established the CASCARA UK-based pilot study (see Panel 19, page 30), which provides a snapshot of the economic burden and supportive care needs for people affected by breast cancer. Nearly all of the 606 people living with breast cancer and carers surveyed by the Commission stated physical or well-being issues related to breast cancer. “I lost my job when I started chemotherapy as I could not cope very well”, one participant said. “It took me a long time to ask for help with sexual dysfunction”, said another. Additionally, 20% of participants with early breast cancer and 25% of those with MBC reported difficulty in covering the costs of travel for treatment. 27% with early breast cancer and 35% with MBC said they had financial problems. This pilot research suggests that, even in countries with a health care system free at the point of care, people with breast cancer can incur hidden costs.
Building on previous work [1], the Commission report also discusses serious health-related suffering (SHS), an indicator of the need for palliative care. Estimates of SHS in breast cancer were provided by a small expert group. Based on the reported 685,000 global breast cancer deaths in 2020, an estimated 120 million days were spent with serious health-related suffering per year for people who died of their cancer. A further 520 million days were estimated for patients living with the disease. Behind these numbers are individuals experiencing pain, shortness of breath, fatigue, and other, often resolvable, distressing symptoms.
“The impact of breast cancer is wide-reaching, and the studies included in our report hint at the enormity of related suffering and negative experiences at all disease stages. Even in countries with well-developed health care systems, patients with breast cancer experience inadequate support and care. In countries lacking affordable health care facilities, patients experience these costs more commonly and intensely, too often leading to catastrophic spending and impoverishment. Global data are essential to expose and better understand and address the multiplicity of needs of all people affected by breast cancer and significantly reduce the global burden of preventable suffering”, says Dr Carlos Barrios, Oncology Research Center, Hospital São Lucas, Brazil.
The Commission advocates the development of new tools and metrics that capture the many costs associated with the disease. This measurement should guide policymakers to invest in breast cancer prevention, early detection, cost-effective therapy, optimal management, financial protection, and other interventions that relieve suffering.
Better communication for better patient outcomes
Breast cancer is a disease that many patients describe as robbing them of power. Therefore, healthcare professional-patient communication that empowers is highlighted by the authors as an important intervention. A review of research by the Commission suggests better communication with patients can improve quality of life, decision-making, body image, and even adherence to therapy – with positive impacts on survival.
“Women’s fundamental human rights have historically been accorded lesser respect than men’s in all settings, with implications for patient agency and autonomy”, says Professor Reshma Jagsi, Emory University School of Medicine, USA. She continues: “Every healthcare professional should receive some form of communication skills training. Improving the quality of communication between patients and health professionals, though seemingly simple, could have profound positive impacts that extend far beyond the specific setting of breast cancer management. Patients should be encouraged to exercise their voices, choosing their level of involvement in care decisions.”
The Commission calls for 100% of healthcare professionals in 100% of countries to receive communication skills training and for patient involvement in all stages of clinical research on breast cancer – from concept to translation into clinical practice. To support these transitions, the report outlines a framework based on strategies to build rapport and empathy, share information, check understanding, and jointly agree next steps with patients.
Collaborate to improve prevention and early detection
Up to one-quarter of breast cancer in HICs could be prevented by modifying risk factors for breast cancer. While education and awareness-raising efforts are important in this respect, bold policy changes that minimise population exposure to modifiable risk factors (including alcohol consumption, being overweight, and physical inactivity) are vital to reduce cancer incidence, suggests the Commission.
In addition, systematic approaches that identify those at increased risk of the disease are essential to enable equitable access to personalised prevention strategies, including cheap and effective medications that can avert breast cancer for many women.
The authors also argue for improved early detection programs, beginning with efforts to promote stage shifting in diagnosis so that at least 60% of confirmed invasive cancers are early disease (stages one or two).
“In all countries, women with low incomes from minoritised backgrounds often have breast cancer diagnosed at a late stage, with a higher risk of dying. Our research lays out numerous other inequities in breast cancer that are at risk of widening further, and that can be addressed through global collaboration. Access to evidence-based prevention and care that isn’t dependent on where an individual lives or their ability to pay would reap wide-ranging benefits for patients, families, and health care systems striving to achieve universal health coverage. We urge decision-makers to implement our recommendations and accelerate progress on closing the breast cancer equity gap”, says Professor Benjamin Anderson, Departments of Surgery and Global Health, University of Washington, USA, who as medical officer at the World Health Organization from 2021 to 2023 oversaw the development and launch of WHO’s Global Breast Cancer Initiative (GBCI).
NOTES TO EDITORS
A full list of Commission authors and their institutions is available in the report.
All quotes in the press release come directly from authors or patient advocates and cannot be found in the text of the Commission.
Funding and support for the Commission has been provided by the National Institute for Health and Care Research (NIHR), Breast Cancer Now, ABC Global Alliance, and the University of Cambridge. Further information about funders and their roles can be found in the Commission report.
[1] Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief–an imperative of universal health coverage: the Lancet Commission report. Lancet 2018; 391: 1391–454. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)32513-8/fulltext
Journal
The Lancet
DOI
10.1016/S0140-6736(24)00747-5
Method of Research
Literature review
Subject of Research
People
Article Title
The Lancet Breast Cancer Commission
Article Publication Date
15-Apr-2024
COI Statement
CEC reports grants from Breast Cancer Now, Cancer Research UK
(CRUK), Addenbrooke’s Charitable Trust, and the National Institute for
Health and Care Research (NIHR); participation in the Independent
Data Monitoring Committees for the UK PivotalBoost trial (chair),
the PROTIS phase III sinonasal proton versus IMRT trial (member),
the TORPEdO Proton Beam Therapy trial (member); and a leadership
role for the Lancet Breast Cancer Commission (chair). CHB reports
grants and research support from AbbVie, Nektar Therapeutics, Pfizer,
Polyphor, Amgen, Daiichi Sankyo, Sanofi, Exelixis, Regeneron, Novartis,
Henlius, Shanghai Henlius Biotech, GSK, Janssen, OBI Pharma,
Eli Lilly, Seagen, Checkpoint Therapeutics, Roche, Bristol Myers Squibb,
MSD, Merck Serono, AstraZeneca, Novocure, Aveo Oncology, Takeda
Pharmaceuticals, TRIO Pharmaceuticals, PharmaMar, Celgene,
Myovant, PPD, Syneos Health, DOCS, Labcorp, ICON, IQVIA, Parexel,
Nuvisan, PSI, and Medpace; ownership or stocks in Tummi and
MEDSir; and participation in advisory boards and consulting activities
for Boehringer Ingelheim, GSK, Novartis, Pfizer, Genentech, Eisai,
Bayer, MSD, AstraZeneca, Zodiac, Eli Lilly, Sanofi, and Daiichi Sankyo.
DAC reports research grants and support from Exact Sciences and
Novartis; consulting fees from Eli Lilly, Novartis, Seagen, Daiichi
Sankyo, Erytech Pharma, Carnall Farrar, Sapience, and Sanofi; payment
or honoraria for lectures and presentations from Exact Sciences, Gilead,
Eli Lilly, and Pfizer; participation on data safety monitoring or advisory
boards for Novartis, AstraZeneca, and Grail; unremunerated leadership
roles for Make Seconds Count (chair of charity trustees) and the Breast
International Group (chair of board of not-for-profit research
organisation); and receipt of funding for analysis and medical writing
for Eli Lilly and Novartis. FC reports payment or honoraria for lectures
and presentations and support for attending meetings and travel from
Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi Sankyo,
Eisai, GE Oncology, Genentech, Gilead, GSK, IQVIA, Macrogenics,
Medscape, MSD, Merus, Mylan, Mundipharma, Novartis, Pfizer,
Pierre Fabre, prIME Oncology, Roche, Sanofi, Samsung Bioepis, Seagen,
Teva Pharmaceuticals, and Touch Independent Medical Education;
institutional financial support for clinical trials from Amgen,
AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb,
Daiichi Sankyo, Eisai, Fresenius, Genentech, Gilead, GSK, Ipsen, Incyte,
Nektar Therapeutics, Nerviano, Novartis, Macrogenics, MediGene,
MedImmune, MSD, Millenium, Pfizer, Pierre Fabre, Roche, Sanofi,
Sonus, Tesaro, Tigris, Wilex, and Wyeth; a leadership role for the ABC
Global Alliance and ABC Consensus Conference and Guidelines
(president); and membership of ESMO, ESO, EORTC-BCG, IBCSG,
SOLTI, ASCO, AACR, EACR, SIS, and ASPIC. WC reports honoraria
from AstraZeneca, Pfizer, MSD, and Eisai. PAF reports a former role in
the scientific advisory committee of Breast Cancer Trials Australia and
New Zealand cooperative group (chair). RJ reports support from the
Susan G Komen Foundation; grants from the National Institutes of
Health (NIH), the Doris Duke Charitable Foundation, the Greenwall
Foundation, and the American Cancer Society; serving as an expert
witness for Kleinbard and Hawks Quindel Law; roles in the Board of
Directors of ASCO (former member), ASTRO’s Ethics Committee
(chair), and the Women in Radiation Oncology Affinity Group (chair);
membership on the Medical Advisory Board for Blue Cross Blue Shield
Association; stock options for their advisory board role in Equity
Quotient; and personal fees from the NIH, Doris Duke Charitable
Foundation, and the Greenwall Foundation. FMK reports research
grants from the ABC Global Alliance, Breast Cancer Now, Merck Serono
WHO, MSD, Avon Cosmetics, the US Cancer Pain Relief Committee,
and the Medical Research Council; consulting fees from Merck Serono
and Instituto Tecnológico y de Estudios Superiores de Monterrey
Mexico; and leadership roles for the Board of Directors of Women in
Global Health (unpaid member), Tómatelo a Pecho (unpaid president
and founder), the Mexican Health Foundation (unpaid Senior
Economist), and the Board of Directors of the International Association
for Hospice and Palliative Care (unpaid member). SAMcI reports grant
funding from the NIHR, CRUK, and Breast Cancer Now; honoraria
from Roche, MSD, AstraZeneca, Becton, Dickinson and Company; travel
and conference support from Roche, Eli Lilly, and MSD; membership of
advisory boards for Eli Lilly, MSD, Roche, and Novartis; and roles for the
UK National Cancer Research Institute Breast Research Group (unpaid
chair) and the Royal College of Surgeons Breast Surgical Specialty Lead
(unpaid). K-AP reports clinical trial funding from AstraZeneca (paid to
institution) and unremunerated previous participation in AstraZeneca
advisory boards. LR reports grants from the European Commission
Horizon 2020 and the German Research Foundation and a role in the
International Association for Hospice and Palliative Care (Chair of Board
of Directors). MKT reports grants from Addenbrooke’s Charitable Trust
and CRUK. FA reports grants from Novartis, Pfizer, AstraZeneca,
Eli Lilly, Daiichi Sankyo, and Roche and consulting fees from
MedImmune, Gilead, Relay Therapeutics, and Guardant Health.
JEA reports speaker honoraria from AstraZeneca, Pfizer, Novartis, and
Eisai and conference travel support from AstraZeneca. ISB reports
participation in the Roche advisory board for ESMO. JMB reports
research grants from Breast Cancer Now, AstraZeneca, MSD, Puma
Biotechnology, Clovis Oncology, Pfizer, Janssen-Cilag, Novartis, Eli Lilly,
Roche, CRUK, and the NIHR. MAF is funded by a Conquer Cancer
Breast Cancer Research Foundation Career Development Award for
Diversity and Inclusion supported by the Breast Cancer Research
Foundation and reports financial support from the WeShare project.
AF reports consultancy work for Oxford Immune Algorithmics; grants
from Addenbrooke’s Charitable Trust and the Ann McLaren Building
Preclinical Imaging Suite of the University of Cambridge; and travel
support from the University of Cambridge. DIR reports support from
the University of Chicago Center for Global Health. DN reports research
support from Cepheid; a grant from the US National Cancer Institute;
and an unpaid leadership role with the WHO Global Breast Cancer
Initiative. WER reports grants from the Cambia Health Foundation, the
Robert Wood Johnson Foundation, and the Rita and Alex Hillman
Foundation; and book royalties from Springer Publishing and Jones &
Bartlett Learning. JR reports funding from the NIHR and support from
AstraZeneca for attendance at a teaching event. DS reports a leadership
The Lancet Commissions
44 www.thelancet.com Published online April 15, 2024 https://doi.org/10.1016/S0140-6736(24)00747-5
role at the Jamaica Cancer Care and Research Institute (co-director).
HS reports membership of the Breast Cancer Now Tissue Bank Advisory
Board and voluntary patient membership of the Independent Cancer
Patients’ Voice charity; an honorarium from the CRUK Precision Grand
Challenge; and support for travel expenses from Breast Cancer Now,
CRUK, the Association of Breast Surgeons UK, and Breast International
Group. IV-L reports grants from Resilience; consulting fees from
Novartis, AstraZeneca, and Amgen; payment or honoraria for lectures
and presentations from Novartis, Sandoz, Amgen, AstraZeneca, and
Pfizer; and support for travel and attending meetings from Novartis.
CV-G reports grants from AstraZeneca and Roche; consulting fees from
Roche, Novartis, Pfizer, and Eli Lilly; honoraria from Roche, Myriad
Genetics, and Novartis; and support for attending meetings and travel
from Roche, MSD Oncology, and Pfizer. All other authors declare no
competing interests.