New SPECT/CT technique shows impressive biomarker identification, offers increased access for prostate cancer patients
Reston, VA—A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more personalized treatment. Utilizing lead-212 (212Pb), the new imaging technique has the potential to change practice and increase access for patients around the world. The first-in-human images from this method were published in the April issue of The Journal of Nuclear Medicine.
Credit: Dr. Matthew Griffiths from the Royal Brisbane and Women’s Hospital developed the SPECT imaging protocol, and the clinical team from the Princess Alexandra Hospital, Brisbane acquired the imaging data.
Reston, VA—A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more personalized treatment. Utilizing lead-212 (212Pb), the new imaging technique has the potential to change practice and increase access for patients around the world. The first-in-human images from this method were published in the April issue of The Journal of Nuclear Medicine.
There is significant interest in the development of 212Pb-PSMA–based targeted alpha therapy (TAT) for patients with metastatic castration-resistant prostate cancer. However, 212Pb is a challenging isotope to image because of the high-energy gamma rays generate significant scatter.
“The ability to acquire imaging of an alpha-emitter with a standard SPECT camera and standard collimator within a convenient acquisition time for the patient could provide more precision in how we treat patients with prostate cancer, and patients with other cancers, in the future. Confirming the presence of the drug in the target is important because it serves as a quality assurance and can be used to derive an understanding of the biodistribution and pharmacokinetics of the drug,” said Stephen Rose, PhD, head of Translational Medicine and Clinical Science at AdvanCell.
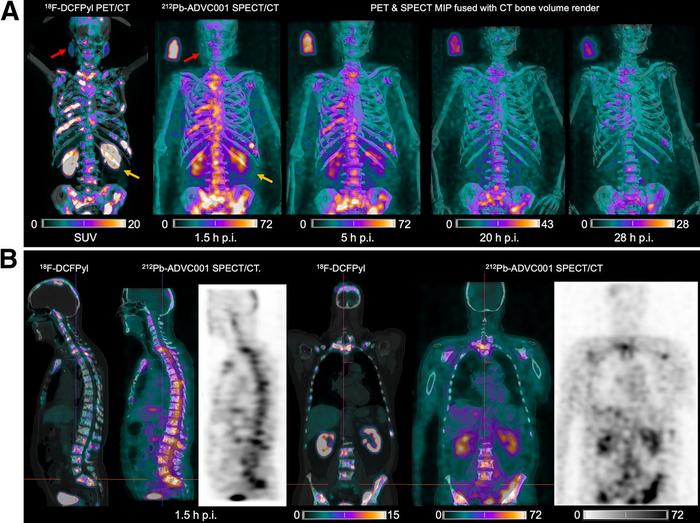
In the study, researchers administered 60 MBq of 212Pb-ADVC001 to a 73-year-old man with metastatic castration-resistant prostate cancer. SPECT/CT imaging occurred at 1.5, 5, 20, and 28 hours after infusion.
Representative 212Pb SPECT/CT images showed rapid tumor uptake of 212Pb-ADVC001 in agreement with tumor burden shown on the pretreatment 18F-DCFPyl PET/CT images. Images acquired after 20 hours showed persistent tumor uptake despite low counts due to 212Pb decay.
“In the future, this imaging technique can help to streamline the drug development process, driving conviction in the agents we bring to larger scale trials. In addition, the ability to image 212Pb with a standard SPECT camera in a relatively short timeframe means that 212Pb is a true theranostic alpha-emitter and could be a valuable in selecting patients for targeted alpha-therapies,” said Rose
He continued, “What’s more, access to PET imaging is a bottleneck, in the United States and globally. SPECT cameras are more widely available and may address this critical issue, as SPECT imaging can be used for patient selection, therapy decision making, and guiding adaptive dosing strategies based on changes of target expression and tumor volume during treatment.”
The study was published online in February 2024.
The authors of “First-in-Human 212Pb-PSMA–Targeted α-Therapy SPECT/CT Imaging in a Patient with Metastatic Castration-Resistant Prostate Cancer” include Matthew R. Griffiths, David A. Pattison, and Melissa Latter, Department of Nuclear Medicine and Specialist PET Services, Royal Brisbane and Women’s Hospital, Brisbane, Queensland, Australia; Kevin Kuan, Stephen Taylor, William Tieu, Thomas Kryza, Danielle Meyrick Boon Quan Lee, Stephen E. Rose, and Simon G. Puttick, AdvanCell, Sydney, New South Wales, Australia; and Aaron Hansen, Department of Medical Oncology, Princess Alexandra Hospital, Brisbane, Queensland, Australia.
Visit the JNM website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed or follow us on LinkedIn.
###
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected].
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed more than 16 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit www.snmmi.org
Journal
Journal of Nuclear Medicine
DOI
10.2967/jnumed.123.267189
Article Title
First-in-Human 212Pb-PSMA–Targeted α-Therapy SPECT/CT Imaging in a Patient with Metastatic Castration-Resistant Prostate Cancer
Article Publication Date
1-Apr-2024
COI Statement
This work was financially supported by AdvanCell through the TheraPb clinical trial (NCT05720130). Kevin Kuan, Stephen Taylor, William Tieu, Thomas Kryza, Stephen Rose, and Simon Puttick are AdvanCell employees. Danielle Meyrick and Boon Quan Lee are AdvanCell consultants.