Repurposed cancer drug could treat diabetes by nudging pancreatic acinar cells to produce insulin
In 2016, University of Pittsburgh researchers Dr. Farzad Esni, Ph.D., and Jing Hu, Ph.D., did an experiment in mice where they deleted one of two copies of the gene encoding an enzyme called focal adhesion kinase (FAK). They were interested in the role of FAK in pancreatic cancer, but a surprise finding took the research in a very different direction.
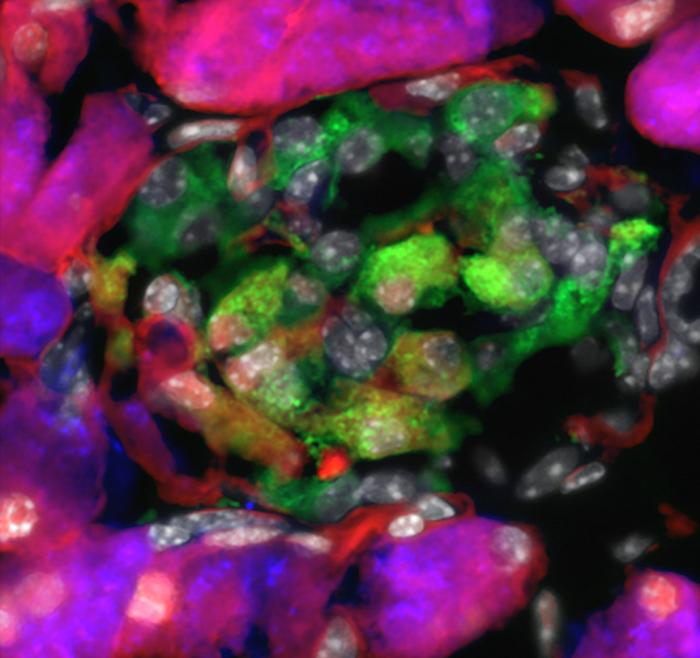
Credit: Farzad Esni
In 2016, University of Pittsburgh researchers Dr. Farzad Esni, Ph.D., and Jing Hu, Ph.D., did an experiment in mice where they deleted one of two copies of the gene encoding an enzyme called focal adhesion kinase (FAK). They were interested in the role of FAK in pancreatic cancer, but a surprise finding took the research in a very different direction.
“The pancreas looked weird, almost like it was trying to regenerate after an injury,” said Esni, associate professor of surgery at Pitt and member of UPMC Hillman Cancer Center and the McGowan Institute for Regenerative Medicine.
Even weirder, a cluster of cells in the pancreas were expressing both insulin and amylase. In normal mice and humans, the blood sugar-regulating hormone insulin is produced by beta cells, while amylase, a digestive enzyme, is manufactured by acinar cells. The functions of acinar and beta-cells are very distinct, so it didn’t make sense that the cluster of cells looked like a combination of the two.
“There were three possible explanations for what we saw in the mutant mice,” said Esni. “It could have just been an artifact of our experiment, beta cells could have started making amylase or acinar cells could have started producing insulin — which would be the holy grail.”
Esni and his team had in fact stumbled upon this holy grail. In a new Nature Communications paper, the researchers show that a FAK-inhibiting drug, which has been studied in cancer treatment, converted acinar cells into acinar-derived insulin-producing (ADIP) cells and helped regulate blood glucose in diabetic mice and a single non-human primate.
The findings suggest that FAK inhibitors could be a new avenue as a replacement for insulin therapy in diabetic patients. Without enough insulin, patients with diabetes are at risk for hyperglycemia, or high blood sugar, which can damage blood vessels and organs and lead to heart attacks, stroke and other serious complications.
To investigate the effects of ADIP cells in an animal model of diabetes, the researchers partially or completely wiped out the animals’ beta cells with a low or high dose of a compound called streptozotocin, mimicking diabetes. Then they treated the mice with a 3-week course of an oral FAK-inhibiting drug called PF562271 or placebo.
The FAK inhibitor-treated mice regained about 30% of their original beta cell mass, and the treatment partially improved hyperglycemia. These results persisted until the end of the experiment several weeks later, suggesting that a one-time treatment may have long-term benefits for diabetes control.
The team also examined the effects of FAK inhibitor in a single non-human primate. After four macaques received diabetes-inducing streptozotocin, they required 5 to 20 units of insulin per day to manage their blood glucose. Next, the researchers treated one of these diabetic macaques with a 3-week course of FAK inhibitor. Six weeks later, the animal’s insulin requirements decreased by 60%, a stable improvement that continued without additional treatment until the end of the experiment four months later.
The idea of nudging acinar cells to produce insulin isn’t new, but FAK inhibitors may have a smoother translational path than genetic approaches because the drug has already been tested in phase 1 cancer trials. It’s also given orally, which is simpler than complicated genetic tools that involve viral delivery of foreign genes or genetic factors that switch on genes.
“Functionally, ADIP cells should be similar to insulin-producing cells derived from acinar cells in other studies, but an important distinction is that our cells actually infiltrated pre-existing pancreatic islets, where beta cells normally reside,” said Esni. “Our cells can take advantage of the islet environment, where they have access to blood vessels for glucose monitoring, which makes them much more potent.”
With the eventual hope of launching a clinical trial to test FAK inhibitor in diabetes patients, Esni and his team are now planning long-term experiments in mice to look at the duration of hyperglycemia control after a single course of the drug in mouse models for type 1 or type 2 diabetes. They’re also investigating the effects of FAK inhibition in pancreatic tissues from human donors.
Other authors on the study were Shakti Dahiya, Ph.D., Mohamed Saleh, M.D., Uylissa A. Rodriguez, Ph.D., Dhivyaa Rajasundaram, Ph.D., Jorge R. Arbujas, Arian Hajihassani, Anuradha Sehrawat, Ph.D., Ranjeet Kalsi, D.O., Shiho Yoshida, M.D., Krishna Prasadan, Ph.D., Jon D. Piganelli, Ph.D., and George K. Gittes, M.D., all of Pitt and UPMC; Kaiyuan Yang, Ph.D., of the Institute of Diabetes and Regeneration Research and the German Center for Diabetes Research; and Heiko Lickert, Ph.D., of the Institute of Diabetes and Regeneration Research, the German Center for Diabetes Research and the Technical University of Munich.
This research was supported by the National Institutes of Health (NIH; R01DK101413, R01DK120698, R21AI158824, CA236965 and 1K08DK129834), JDRF (2-SRA-2022-1211-S-B), Cochrane-Weber Endowed Funds for Diabetes Research, UPMC Children’s Hospital of Pittsburgh and the University of Pittsburgh Center for Research Computing (NIH award S10OD028483).
Journal
Nature Communications
DOI
10.1038/s41467-024-47972-4
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Acinar to β-like cell conversion through inhibition of focal adhesion kinase
Article Publication Date
3-May-2024