Road of no return — loss of TP53 paves a defined evolution path from gastric preneoplasia-to-cancer
“The independent research groups, led by Prof. Scott W. Lowe and Christina Curtis,respectively, have uncovered a similar definitive pathway in the progression of gastric cancer (GC) initiated with loss of the TP53 gene, representing a milstone in understanding the early stages of this deadly disease”. Dr. Zhaocai Zhou, head of a GC laboratory from Fudan University, stated.“Their study offers detailed insights into how genetic changes drive the transformation from preneoplastic conditions to full-blown cancer. Their findings revealed that loss of TP53 is not merely a common genetic anomaly but a pivotal event that propels the cellular evolution from benign to malignant states in a manner that is not totally random. This discovery opens new possibility to prevent and treat GC.”
GC ranks as the fifth most common and fourth deadliest cancer worldwide, presenting significant health challenges, particularly in Chinawhere it is most prevalent and accounts for nearly half of newly diagnosised and death cases. In addition to somatic mutantions, the complex pathology of GC is also determined by exposure to external factors like dietary and microorganisms such as Helicobacter pylori,Streptococcus anginosus, andCandida albicans.Thus, a long-standing open question in this field is “how the intrinsic genetic driver mutations such as loss of TP53coordinate with extrinsic risk factors such as microbial infection to initiate the gastric tumorigenesis”.
A recent editorial contributed by Dr. Zhaocai Zhou (DOI:10.20892/j.issn.2095-3941.2023.0435), published in December 2023 in Cancer Biology & Medicine, systemically digested the work published by the Lowe’s and Curtis’ groups in Nature, which sheds light on the critical role of TP53 loss,a gene known for its tumor-suppressing functions, in drving GC initiation; and discussed the limitations and future directions of this burgeoning research area.
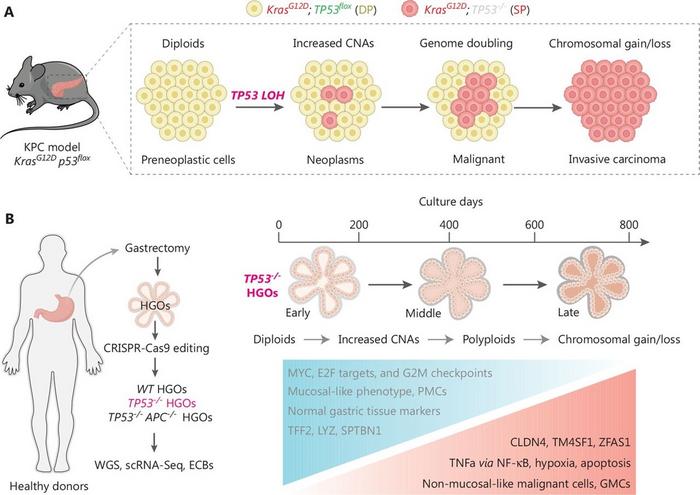
Using innovative animal models and cutting-edge genomic technologies, the researchers were able to track cellular transformations in real time. Their key findings indicate that loss of TP53 gene function initially leads to an increase in chromosomal instability. This early instability, marked by widespread genetic rearrangements, increases the propensity for aggressive cancer traits to develop. As the disease progresses, specific gene alterations, including changes in gene copy numbers and structural variationsoccur in a relatively defined but not random order, which are crucial for the cancer to reach its full malignant potential. Importantly, the two groups identified distinct phases of genetic evolution, all linked to the worsening of the disease. The initial phase involves subtle genomic changes, followed by more pronounced genetic alterations that solidify the cancer’s growth and spread. This progression pattern offers potential markers for early detection and inspirespreventive or therapeutic strategies aiming to interrupt this deadly evolution path.
“Understanding the role of TP53 loss in the roadmap of GC pathology opens new avenues for early detection and intervention of GC. By identifying the genetic trajectory of tumor evolution, clinicians can potentially devise strategies to intercept the road to cancer much earlier. Moreover, these findings support the use of personalized medicine approaches tailored to the genetic profile of individual tumors.” Dr. Zhou further addressed, “In this regard, an immediateprominent issue is to evaluate how extrinsic factors influence TP53 loss-related evolution path during GC initiation and development; and future studies might rediscoverthe extrinsic factors in a second-hit paradigm”.
Credit: Cancer Biology & Medicine
“The independent research groups, led by Prof. Scott W. Lowe and Christina Curtis,respectively, have uncovered a similar definitive pathway in the progression of gastric cancer (GC) initiated with loss of the TP53 gene, representing a milstone in understanding the early stages of this deadly disease”. Dr. Zhaocai Zhou, head of a GC laboratory from Fudan University, stated.“Their study offers detailed insights into how genetic changes drive the transformation from preneoplastic conditions to full-blown cancer. Their findings revealed that loss of TP53 is not merely a common genetic anomaly but a pivotal event that propels the cellular evolution from benign to malignant states in a manner that is not totally random. This discovery opens new possibility to prevent and treat GC.”
GC ranks as the fifth most common and fourth deadliest cancer worldwide, presenting significant health challenges, particularly in Chinawhere it is most prevalent and accounts for nearly half of newly diagnosised and death cases. In addition to somatic mutantions, the complex pathology of GC is also determined by exposure to external factors like dietary and microorganisms such as Helicobacter pylori,Streptococcus anginosus, andCandida albicans.Thus, a long-standing open question in this field is “how the intrinsic genetic driver mutations such as loss of TP53coordinate with extrinsic risk factors such as microbial infection to initiate the gastric tumorigenesis”.
A recent editorial contributed by Dr. Zhaocai Zhou (DOI:10.20892/j.issn.2095-3941.2023.0435), published in December 2023 in Cancer Biology & Medicine, systemically digested the work published by the Lowe’s and Curtis’ groups in Nature, which sheds light on the critical role of TP53 loss,a gene known for its tumor-suppressing functions, in drving GC initiation; and discussed the limitations and future directions of this burgeoning research area.
Using innovative animal models and cutting-edge genomic technologies, the researchers were able to track cellular transformations in real time. Their key findings indicate that loss of TP53 gene function initially leads to an increase in chromosomal instability. This early instability, marked by widespread genetic rearrangements, increases the propensity for aggressive cancer traits to develop. As the disease progresses, specific gene alterations, including changes in gene copy numbers and structural variationsoccur in a relatively defined but not random order, which are crucial for the cancer to reach its full malignant potential. Importantly, the two groups identified distinct phases of genetic evolution, all linked to the worsening of the disease. The initial phase involves subtle genomic changes, followed by more pronounced genetic alterations that solidify the cancer’s growth and spread. This progression pattern offers potential markers for early detection and inspirespreventive or therapeutic strategies aiming to interrupt this deadly evolution path.
“Understanding the role of TP53 loss in the roadmap of GC pathology opens new avenues for early detection and intervention of GC. By identifying the genetic trajectory of tumor evolution, clinicians can potentially devise strategies to intercept the road to cancer much earlier. Moreover, these findings support the use of personalized medicine approaches tailored to the genetic profile of individual tumors.” Dr. Zhou further addressed, “In this regard, an immediateprominent issue is to evaluate how extrinsic factors influence TP53 loss-related evolution path during GC initiation and development; and future studies might rediscoverthe extrinsic factors in a second-hit paradigm”.
###
References
DOI
10.20892/j.issn.2095-3941.2023.0435
Original Source URL
https://doi.org/10.20892/j.issn.2095-3941.2023.0435
Funding information
This work was supported by the National Key R&D Program of China (Grant Nos. 2020YFA0803200 and 2017YFA0504504), the National Natural Science Foundation of China (Grant Nos. 82222052 and 32070710), the Science and Technology Commission of Shanghai Municipality (Grant Nos. 22QA1407300, 23ZR1480400, and 23YF1432900), and Talent Climbing Project from Shanghai Tenth People’s Hospital (Grant Nos. 2021SYPDRC003).
About Cancer Biology & Medicine
Cancer Biology & Medicine (CBM) is a peer-reviewed open-access journal sponsored by China Anti-cancer Association (CACA) and Tianjin Medical University Cancer Institute & Hospital. The journal monthly provides innovative and significant information on biological basis of cancer, cancer microenvironment, translational cancer research, and all aspects of clinical cancer research. The journal also publishes significant perspectives on indigenous cancer types in China. The journal is indexed in SCOPUS, MEDLINE and SCI (IF 5.5, 5 year IF 6.1), with all full texts freely visible to clinicians and researchers all over the world (http://www.ncbi.nlm.nih.gov/pmc/journals/2000/).
DOI
10.20892/j.issn.2095-3941.2023.0435
Subject of Research
Not applicable
Article Title
Road of no return — loss of TP53 paves a defined evolution path from gastric preneoplasia-to-cancer
Article Publication Date
12-Dec-2023
COI Statement
The authors declare that they have no competing interests.