
Supercharging immune cells to battle blood cancer: Breakthrough in cancer immunotherapy
A new study reveals a groundbreaking approach to immunotherapy, demonstrating that blocking the interaction between the CD300A receptor and phosphatidylserine (PS) significantly enhances the ability of human natural killer (NK) cells to lyse hematologic malignancies (HMs).
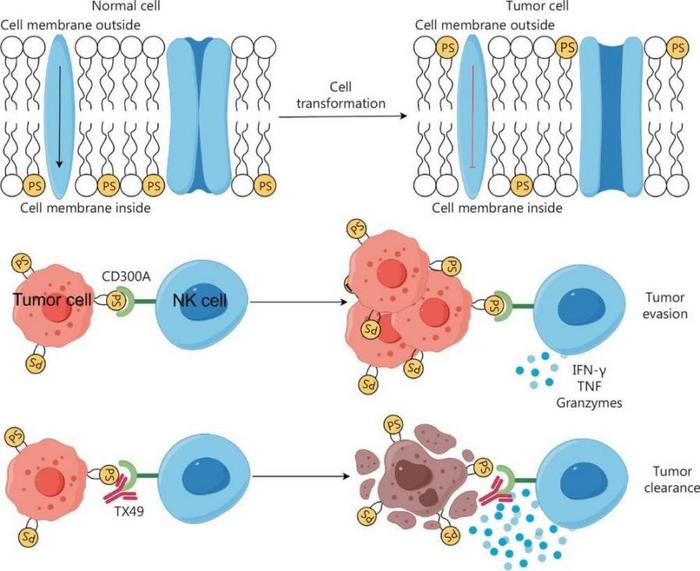
Cancer has a profound impact on human life, and immune checkpoint therapy (ICT) has made remarkable strides in cancer treatment. However, ICT faces challenges such as low overall response rates and the emergence of immune-related adverse events. To overcome these hurdles, researchers are exploring new immune checkpoints. CD300A, a type-I transmembrane protein with immunoreceptor tyrosine-based inhibitory motifs, emerges as a potential immune checkpoint, negatively regulating NK cell function through its interaction with PS.
In a recent study (DOI:10.20892/j.issn.2095-3941.2023.0341) published in Cancer Biology & Medicine, researchers from the University of Science and Technology of China have discovered a novel strategy to enhance the ability of human natural killer (NK) cells to combat hematologic malignancies. By blocking the CD300A protein, a type-I transmembrane protein with immunoreceptor tyrosine-based inhibitory motifs, the team demonstrated a significant boost in the lytic function of NK cells against cancer cells.
The study centered on the CD300A protein, a known immune checkpoint found on the surface of NK cells. The researchers discovered that when CD300A interacts with phosphatidylserine (PS), a molecule present on the surface of cancer cells, it dampens the NK cells’ ability to attack and destroy these cells. To counteract this, the team developed a strategy to block CD300A using a specially designed monoclonal antibody called TX49. By doing so, they were able to enhance the NK cells’ cytotoxic capabilities, making them more effective at lysing cancer cells both in vitro and in vivo. The study demonstrated that blocking CD300A not only increased the expression of proteins related to cell lysis but also the secretion of cytokines, which are crucial for immune response coordination. This approach led to a significant improvement in the survival rate of mice with xenografted human blood cancer cells, offering a promising therapeutic avenue for patients suffering from hematologic malignancies and potentially other types of cancer.
“By targeting CD300A, we can potentially enhance the anti-tumor function of NK cells,” said Zhigang Tian, a lead researcher in the study. “This could open new avenues for treating hematologic malignancies and possibly other cancer types.”
The research suggests that targeting CD300A could be a promising addition to immune checkpoint-based cancer immunotherapy. The ability to invigorate NK cell-based treatments against HMs offers new hope for patients with these challenging cancers. The findings also indicate that enhanced expression of CD300A is associated with shorter survival and a more “exhausted” phenotype of intratumoral NK cells in patients with HMs or solid tumors.
Credit: Cancer Biology & Medicine
A new study reveals a groundbreaking approach to immunotherapy, demonstrating that blocking the interaction between the CD300A receptor and phosphatidylserine (PS) significantly enhances the ability of human natural killer (NK) cells to lyse hematologic malignancies (HMs).
Cancer has a profound impact on human life, and immune checkpoint therapy (ICT) has made remarkable strides in cancer treatment. However, ICT faces challenges such as low overall response rates and the emergence of immune-related adverse events. To overcome these hurdles, researchers are exploring new immune checkpoints. CD300A, a type-I transmembrane protein with immunoreceptor tyrosine-based inhibitory motifs, emerges as a potential immune checkpoint, negatively regulating NK cell function through its interaction with PS.
In a recent study (DOI:10.20892/j.issn.2095-3941.2023.0341) published in Cancer Biology & Medicine, researchers from the University of Science and Technology of China have discovered a novel strategy to enhance the ability of human natural killer (NK) cells to combat hematologic malignancies. By blocking the CD300A protein, a type-I transmembrane protein with immunoreceptor tyrosine-based inhibitory motifs, the team demonstrated a significant boost in the lytic function of NK cells against cancer cells.
The study centered on the CD300A protein, a known immune checkpoint found on the surface of NK cells. The researchers discovered that when CD300A interacts with phosphatidylserine (PS), a molecule present on the surface of cancer cells, it dampens the NK cells’ ability to attack and destroy these cells. To counteract this, the team developed a strategy to block CD300A using a specially designed monoclonal antibody called TX49. By doing so, they were able to enhance the NK cells’ cytotoxic capabilities, making them more effective at lysing cancer cells both in vitro and in vivo. The study demonstrated that blocking CD300A not only increased the expression of proteins related to cell lysis but also the secretion of cytokines, which are crucial for immune response coordination. This approach led to a significant improvement in the survival rate of mice with xenografted human blood cancer cells, offering a promising therapeutic avenue for patients suffering from hematologic malignancies and potentially other types of cancer.
“By targeting CD300A, we can potentially enhance the anti-tumor function of NK cells,” said Zhigang Tian, a lead researcher in the study. “This could open new avenues for treating hematologic malignancies and possibly other cancer types.”
The research suggests that targeting CD300A could be a promising addition to immune checkpoint-based cancer immunotherapy. The ability to invigorate NK cell-based treatments against HMs offers new hope for patients with these challenging cancers. The findings also indicate that enhanced expression of CD300A is associated with shorter survival and a more “exhausted” phenotype of intratumoral NK cells in patients with HMs or solid tumors.
###
References
DOI
10.20892/j.issn.2095-3941.2023.0341
Original Source URL
https://doi.org/10.20892/j.issn.2095-3941.2023.0341
Funding information
This work was supported by the National Key R&D Program of China (2019YFA0508502/3 and 2021YFC2300604), the Natural Science Foundation of China (Reference numbers 82388201, 82241216, and 32270963), the Research Funds of Center for Advanced Interdisciplinary Science and Biomedicine of IHM (QYZD20220008), and the Anhui Key Research and Development Plan (Reference number 2023z04020011).
About Cancer Biology & Medicine
Cancer Biology & Medicine (CBM) is a peer-reviewed open-access journal sponsored by China Anti-cancer Association (CACA) and Tianjin Medical University Cancer Institute & Hospital. The journal monthly provides innovative and significant information on biological basis of cancer, cancer microenvironment, translational cancer research, and all aspects of clinical cancer research. The journal also publishes significant perspectives on indigenous cancer types in China. The journal is indexed in SCOPUS, MEDLINE and SCI (IF 5.5, 5 year IF 6.1), with all full texts freely visible to clinicians and researchers all over the world (http://www.ncbi.nlm.nih.gov/pmc/journals/2000/).
Journal
Cancer Biology & Medicine
DOI
10.20892/j.issn.2095-3941.2023.0341
Subject of Research
Not applicable
Article Title
Blockade of CD300A enhances the ability of human NK cells to lyse hematologic malignancies
Article Publication Date
29-Feb-2024
COI Statement
The authors declare that they have no competing interests.