
Brain “assembloids” mimic human blood-brain barrier
In a pioneering achievement, a research team led by experts at Cincinnati Children’s have developed the world’s first human mini-brain that incorporates a fully functional blood-brain barrier (BBB).
Credit: Cincinnati Children’s and Cell Stem Cell
In a pioneering achievement, a research team led by experts at Cincinnati Children’s have developed the world’s first human mini-brain that incorporates a fully functional blood-brain barrier (BBB).
This major advance, published May 15, 2024, in Cell Stem Cell, promises to accelerate the understanding and improved treatment of a wide range of brain disorders, including stroke, cerebral vascular disorders, brain cancer, Alzheimer’s disease, Huntington disease, Parkinson’s disease, and other neurodegenerative conditions.
“Lack of an authentic human BBB model has been a major hurdle in studying neurological diseases,” says lead corresponding author Ziyuan Guo, PhD, “Our breakthrough involves the generation of human BBB organoids from human pluripotent stem cells, mimicking human neurovascular development to produce a faithful representation of the barrier in growing, functioning brain tissue. This is an important advance because animal models we currently use in research do not accurately reflect human brain development and BBB functionality.”
What is the blood-brain barrier?
Unlike the rest of our bodies, blood vessels in the brain feature an extra lining of tightly packed cells that sharply limit the size of molecules that can pass from the bloodstream into the central nervous system (CNS).
A properly functioning barrier maintains brain health by preventing the entry of harmful substances while allowing essential nutrients to reach the brain. However, that same barrier also prevents many potentially helpful medicines from reaching the brain. Also, several neurological disorders are caused, or worsened, when the blood-brain barrier forms improperly or begins breaking down.
Significant differences between human and animal brains have resulted in many hopeful new drugs that were developed relying heavily on animal models to fail later when tested in human study participants.
“Now, through stem cell bioengineering, we have developed an innovative platform based on human stem cells that allows us to study the intricate mechanisms governing BBB function and dysfunction. This provides unprecedented opportunities for drug discovery and therapeutic intervention,” Guo says.
Overcoming a long-running challenge
Research teams worldwide have been racing to develop brain organoids—tiny, growing 3D structures that mimic the early steps of brain formation. Unlike cell types grown flat in a lab dish, organoid cells are connected. They self-assemble into spherical forms. Their cells “talk” to each other like human cells normally do during fetal development.
Cincinnati Children’s has been a leader in developing other types of organoids, including the world’s first functional intestine, stomach and esophagus organoids. But until now, no research center had succeeded at making a brain organoid that features the special barrier lining found in human brain blood vessels.
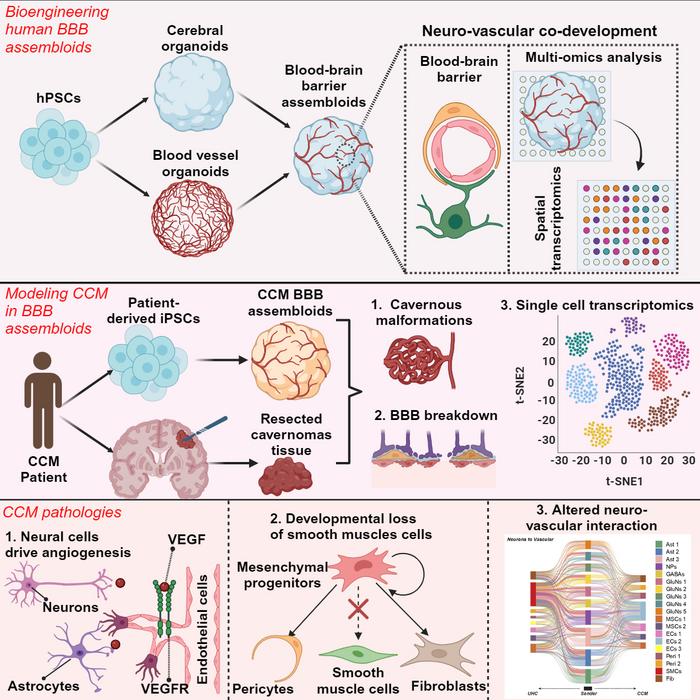
The research team calls their new model “BBB assembloids.” Their name reflects the advance that made the breakthrough possible. These assembloids combine two distinct types of organoids: brain organoids that replicate human brain tissue and blood vessel organoids that mimic vascular structures.
The combination process began with brain organoids measuring 3 to 4 millimeters in diameter and blood vessel organoids about 1 millimeter in diameter. Over the course of about a month, these separate structures fused into a single sphere measuring slightly more than 4 millimeters in diameter (about 1/8 of an inch, or roughly the size of a sesame seed).
These integrated organoids recreate many of the complex neurovascular interactions observed in the human brain, but they are not complete models of the brain. For example, the tissue does not contain immune cells and there are no connections to the rest of the body’s nervous system.
Research teams at Cincinnati Children’s have shown other successes at merging and layering organoids from different cell types to form more complex “next generation” organoids. Those successes helped inform the new brain organoid work.
Importantly, the BBB assembloids can be grown using neurotypical human stem cells or stem cells from people with specific brain diseases, thus reflecting gene variants and other conditions that can lead to a malfunctioning blood-brain barrier.
Initial proof of concept
To demonstrate the potential utility of the new assembloids, the researcher team used a line of patient-derived stem cells to make assembloids that accurately replicated key features of a rare brain condition called a cerebral cavernous malformation.
This genetic disorder, which is characterized by dysfunctional blood-brain barrier integrity, results in clusters of abnormal blood vessels in the brain that often resemble raspberries in their appearance. The disorder significantly increases risk of stroke.
“Our model accurately recapitulated the disease phenotype, offering new insights into the underlying molecular and cellular pathology of cerebral vascular disorders,” Guo says.
Potential applications
The co-authors envision a variety of potential uses of BBB assembloids:
- Personalized Drug Screening: Patient-derived BBB assembloids could serve as avatars to tailor therapies for patients based on their unique genetic and molecular profiles.
- Disease Modeling: A number of neurovascular disorders, including rare and genetically complex conditions, lack good model systems for research. Success at making BBB assembloids could accelerate development of human brain tissue models for more conditions.
- High-Throughput Drug Discovery: Scaling up assembloid production could allow more accurate, and more rapid analysis of whether potential brain medications can effectively cross the BBB.
- Environmental Toxin Testing: Often based heavily on animal model systems, BBB assembloids could help evaluate the toxic effects of environmental pollutants, pharmaceuticals, and other chemical compounds.
- Immunotherapy Development: Through investigating the role of the BBB in neuroinflammatory and neurodegenerative diseases, the new assembloids could support delivering immune-based therapies to the brain.
- Bioengineering and Biomaterials Research: Biomedical engineers and materials scientists will likely benefit from having a lab model of the BBB to test novel biomaterials, drug delivery vehicles, and tissue engineering strategies.
“Overall, BBB assembloids represent a game-changing technology with broad implications for neuroscience, drug discovery, and personalized medicine,” Guo says.
About the study
In addition to Guo, the co-first authors of the study were: Lan Dao, MS, and Lu Lu, PhD, from Cincinnati Children’s; Tianyang Xu from UC San Diego; and Zhen You from the Mayo Clinic. Co-corresponding authors were Sheng Zhong, PhD, from UC San Diego and L. Frank Huang, PhD, from the Mayo Clinic.
Co-authors from Cincinnati Children’s also included Avijite Kumer Sarkar, PhD, Hui Zhu, PhD, George Yoshida, BA, Yifei Miao, PhD, Sarah Mierke, MD, Srijan Kalva, Mingxia Gu, MD, PhD, and Sudhakar Vadivelu, MD. The Single Cell Genomics Facility at Cincinnati Children’s and the NIH NeuroBioBank also provided key support to the research.
Guo and Dao have a pending patent application (“Vascularized brain organoids having a CCM-like feature and methods of making and use,” U.S. Application no. 63/510,463) related to this research. Zhong is a founder of Genemo, Inc.
In a pioneering achievement, a research team led by experts at Cincinnati Children’s have developed the world’s first human mini-brain that incorporates a fully functional blood-brain barrier (BBB).
This major advance, published May 15, 2024, in Cell Stem Cell, promises to accelerate the understanding and improved treatment of a wide range of brain disorders, including stroke, cerebral vascular disorders, brain cancer, Alzheimer’s disease, Huntington disease, Parkinson’s disease, and other neurodegenerative conditions.
“Lack of an authentic human BBB model has been a major hurdle in studying neurological diseases,” says lead corresponding author Ziyuan Guo, PhD, “Our breakthrough involves the generation of human BBB organoids from human pluripotent stem cells, mimicking human neurovascular development to produce a faithful representation of the barrier in growing, functioning brain tissue. This is an important advance because animal models we currently use in research do not accurately reflect human brain development and BBB functionality.”
What is the blood-brain barrier?
Unlike the rest of our bodies, blood vessels in the brain feature an extra lining of tightly packed cells that sharply limit the size of molecules that can pass from the bloodstream into the central nervous system (CNS).
A properly functioning barrier maintains brain health by preventing the entry of harmful substances while allowing essential nutrients to reach the brain. However, that same barrier also prevents many potentially helpful medicines from reaching the brain. Also, several neurological disorders are caused, or worsened, when the blood-brain barrier forms improperly or begins breaking down.
Significant differences between human and animal brains have resulted in many hopeful new drugs that were developed relying heavily on animal models to fail later when tested in human study participants.
“Now, through stem cell bioengineering, we have developed an innovative platform based on human stem cells that allows us to study the intricate mechanisms governing BBB function and dysfunction. This provides unprecedented opportunities for drug discovery and therapeutic intervention,” Guo says.
Overcoming a long-running challenge
Research teams worldwide have been racing to develop brain organoids—tiny, growing 3D structures that mimic the early steps of brain formation. Unlike cell types grown flat in a lab dish, organoid cells are connected. They self-assemble into spherical forms. Their cells “talk” to each other like human cells normally do during fetal development.
Cincinnati Children’s has been a leader in developing other types of organoids, including the world’s first functional intestine, stomach and esophagus organoids. But until now, no research center had succeeded at making a brain organoid that features the special barrier lining found in human brain blood vessels.
The research team calls their new model “BBB assembloids.” Their name reflects the advance that made the breakthrough possible. These assembloids combine two distinct types of organoids: brain organoids that replicate human brain tissue and blood vessel organoids that mimic vascular structures.
The combination process began with brain organoids measuring 3 to 4 millimeters in diameter and blood vessel organoids about 1 millimeter in diameter. Over the course of about a month, these separate structures fused into a single sphere measuring slightly more than 4 millimeters in diameter (about 1/8 of an inch, or roughly the size of a sesame seed).
These integrated organoids recreate many of the complex neurovascular interactions observed in the human brain, but they are not complete models of the brain. For example, the tissue does not contain immune cells and there are no connections to the rest of the body’s nervous system.
Research teams at Cincinnati Children’s have shown other successes at merging and layering organoids from different cell types to form more complex “next generation” organoids. Those successes helped inform the new brain organoid work.
Importantly, the BBB assembloids can be grown using neurotypical human stem cells or stem cells from people with specific brain diseases, thus reflecting gene variants and other conditions that can lead to a malfunctioning blood-brain barrier.
Initial proof of concept
To demonstrate the potential utility of the new assembloids, the researcher team used a line of patient-derived stem cells to make assembloids that accurately replicated key features of a rare brain condition called a cerebral cavernous malformation.
This genetic disorder, which is characterized by dysfunctional blood-brain barrier integrity, results in clusters of abnormal blood vessels in the brain that often resemble raspberries in their appearance. The disorder significantly increases risk of stroke.
“Our model accurately recapitulated the disease phenotype, offering new insights into the underlying molecular and cellular pathology of cerebral vascular disorders,” Guo says.
Potential applications
The co-authors envision a variety of potential uses of BBB assembloids:
- Personalized Drug Screening: Patient-derived BBB assembloids could serve as avatars to tailor therapies for patients based on their unique genetic and molecular profiles.
- Disease Modeling: A number of neurovascular disorders, including rare and genetically complex conditions, lack good model systems for research. Success at making BBB assembloids could accelerate development of human brain tissue models for more conditions.
- High-Throughput Drug Discovery: Scaling up assembloid production could allow more accurate, and more rapid analysis of whether potential brain medications can effectively cross the BBB.
- Environmental Toxin Testing: Often based heavily on animal model systems, BBB assembloids could help evaluate the toxic effects of environmental pollutants, pharmaceuticals, and other chemical compounds.
- Immunotherapy Development: Through investigating the role of the BBB in neuroinflammatory and neurodegenerative diseases, the new assembloids could support delivering immune-based therapies to the brain.
- Bioengineering and Biomaterials Research: Biomedical engineers and materials scientists will likely benefit from having a lab model of the BBB to test novel biomaterials, drug delivery vehicles, and tissue engineering strategies.
“Overall, BBB assembloids represent a game-changing technology with broad implications for neuroscience, drug discovery, and personalized medicine,” Guo says.
About the study
In addition to Guo, the co-first authors of the study were: Lan Dao, MS, and Lu Lu, PhD, from Cincinnati Children’s; Tianyang Xu from UC San Diego; and Zhen You from the Mayo Clinic. Co-corresponding authors were Sheng Zhong, PhD, from UC San Diego and L. Frank Huang, PhD, from the Mayo Clinic.
Co-authors from Cincinnati Children’s also included Avijite Kumer Sarkar, PhD, Hui Zhu, PhD, George Yoshida, BA, Yifei Miao, PhD, Sarah Mierke, MD, Srijan Kalva, Mingxia Gu, MD, PhD, and Sudhakar Vadivelu, MD. The Single Cell Genomics Facility at Cincinnati Children’s and the NIH NeuroBioBank also provided key support to the research.
Guo and Dao have a pending patent application (“Vascularized brain organoids having a CCM-like feature and methods of making and use,” U.S. Application no. 63/510,463) related to this research. Zhong is a founder of Genemo, Inc.
Journal
Cell Stem Cell
DOI
10.1016/j.stem.2024.04.019
Method of Research
Experimental study
Subject of Research
Lab-produced tissue samples
Article Title
Modeling blood-brain barrier formation and cerebral cavernous malformations in human PSC-derived organoids
Article Publication Date
15-May-2024
COI Statement
Co- authors Guo and Dao have a pending patent application (“Vascularized brain organoids having a CCM-like feature and methods of making and use,” U.S. Application no. 63/510,463) related to this research. Zhong is a founder of Genemo, Inc.