New mechanism of immune evasion in squamous cell carcinoma, offers potential for improved treatment
Recently, a research group led by Dr. JIANG Yanyi from Hefei Institutes of Physical Science of Chinese Academy of Sciences, along with Dr. LIN Dechen’s team at the University of Southern California, USA, revealed the tumor-extrinsic function of master regulator transcription factor-TP63 in promoting immune evasion and affecting immunotherapy efficacy in squamous cancer.
Credit: JIANG Yuan
Recently, a research group led by Dr. JIANG Yanyi from Hefei Institutes of Physical Science of Chinese Academy of Sciences, along with Dr. LIN Dechen’s team at the University of Southern California, USA, revealed the tumor-extrinsic function of master regulator transcription factor-TP63 in promoting immune evasion and affecting immunotherapy efficacy in squamous cancer.
The research results were published in Nature Communications.
Immunotherapy such as anti-PD-1/PD-L1 antibodies has significantly improved outcomes of a subset of advanced squamous cell cancer (SCC) patients. However, low response rate and treatment resistance are common. It is still unclear which patients will benefit the most from immunotherapy and how to design rational combinatorial immunotherapeutic strategies, partly because molecular mechanisms underlying immune evasion of SCC tumors remain ill-defined.
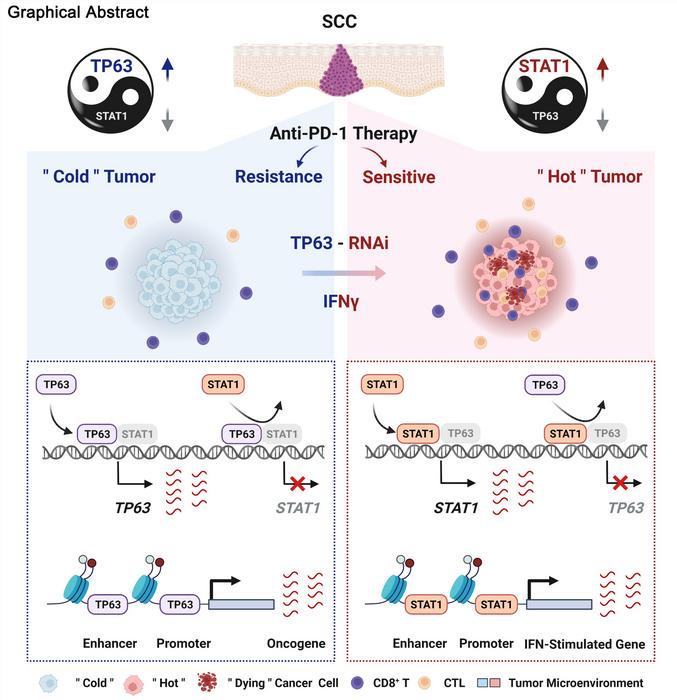
This study started with unbiased high-throughput analyses from 1,077 SCC patient samples and 112 cell lines. Through this, researchers found interferon-γ/α (IFNγ/α) signaling as the most significantly enriched pathway suppressed by TP63, which is often over-expressed specifically in SCCs.
By integration of scRNA-seq, flow cytometry, in vivo syngeneic mouse model and ex vivo co-culture data, the researchers found that CD8+ T cell was the main cell type regulated by TP63. Inhibition of TP63 led to more CD8+ T cell infiltration and heightened tumor killing. In human SCC patients, expression of TP63 was negatively correlated with CD8+ T cell infiltration and activation. Importantly, downregulation of TP63 markedly improved anti-tumor immunotherapy efficacy of PD-1 mAb in SCC mouse models.
Mechanistically, the researchers have revealed that TP63 and STAT1 mutually suppressed each other to regulate the IFNγ signaling by co-occupying and co-regulating their own promoters and enhancers. The relative expression between STAT1 and TP63 dictated the strength of IFNγ signaling, which determined immune response by regulating CD8+ T cell activity.
“Our findings offer new insights into how SCC cells escape immunological surveillance, and suggest targeting IFNγ-TP63/STAT1 axis as a potential strategy to improve anti-tumor immunotherapeutic effect of SCCs”, said Dr. JIANG.
Journal
Nature Communications
Article Title
Reciprocal inhibition between TP63 and STAT1 regulates anti-tumor immune response through interferon-γ signaling in squamous cancer
Article Publication Date
20-Mar-2024