
Identifying the initial steps in colorectal cancer formation
Research led by Weill Cornell Medicine provides new evidence that most colorectal cancers begin with the loss of intestinal stem cells, even before cancer-causing genetic alterations appear. The results, published on May 29 in Developmental Cell, overturn the prevailing theory for colorectal tumor initiation and suggest new ways to diagnose the disease before it has a chance to become established.
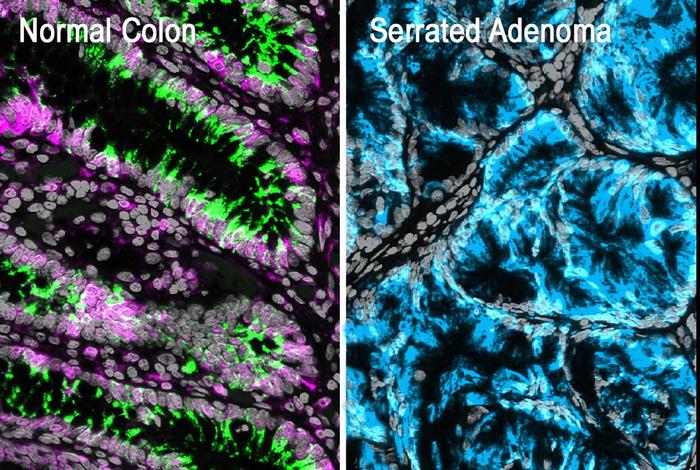
Credit: Moscat Lab
Research led by Weill Cornell Medicine provides new evidence that most colorectal cancers begin with the loss of intestinal stem cells, even before cancer-causing genetic alterations appear. The results, published on May 29 in Developmental Cell, overturn the prevailing theory for colorectal tumor initiation and suggest new ways to diagnose the disease before it has a chance to become established.
“Colorectal cancer is very, very heterogeneous, which has made it difficult for many years to classify these tumors in order to inform therapy,” said senior author Dr. Jorge Moscat, Homer T. Hirst III Professor of Oncology in Pathology and Vice-Chair for Cell and Cancer Pathobiology in the Department of Pathology and Laboratory Medicine at Weill Cornell Medicine. This heterogeneity, the diverse characteristics of colorectal tumor cells in different patients and also within the same tumor, makes treatment particularly challenging.
Colorectal tumors can arise from two types of pre-cancerous polyps: conventional adenomas and serrated adenomas. Conventional adenomas were thought to develop from mutations in the normal stem cells that lie at the bottoms of intestinal crypts, pit-like structures in the lining of the intestine. Serrated adenomas, on the other hand, are associated with a different type of stem-like cell with fetal characteristics that appears mysteriously at the tops of the crypts. Scientists in the field have described these apparently distinct tumor-forming processes as “bottom-up” and “top-down.”
“We wanted to determine how those two routes really start and how they progress, so we can better understand their heterogeneity as the cancer progresses,” said co-senior author Dr. Maria Diaz-Meco, Homer T. Hirst Professor of Oncology in Pathology in the Department of Pathology and Laboratory Medicine at Weill Cornell Medicine and a member of the Meyer Cancer Center at Weill Cornell Medicine. That’s particularly important for serrated tumors, which doctors sometimes miss because of their initial flat shape, and which can become aggressive cancers later.
The co-first authors are Dr. Hiroto Kinoshita and Dr. Anxo Martinez-Ordoñez, postdoctoral associates in the Department of Pathology and Laboratory Medicine at Weill Cornell Medicine.
Getting to the Bottom of Colorectal Cancer
The researchers previously found that many human colorectal tumors of both origins have abnormally low levels of proteins called atypical protein kinase C (aPKC). The new study investigated what happens when the aPKC genes are inactivated in animal models and cultured intestinal organoids.
“We approached this project with the bottom-up and top-down theories, but we were surprised to find that both tumor types showed loss of intestinal stem cells after aPKC genes were inactivated,” said Dr. Moscat, who is also a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine.
The characteristic top-side stem cells on serrated adenomas only arise after the normal stem cells at the bottom of the crypt die, throwing the structure of the entire crypt into disarray. “So, the conventional cancer is bottom-up, and the serrated cancer is also bottom-up,” said Dr. Moscat.
The findings suggest a new unified model for the initiation of colorectal cancer where damage to the intestinal crypts causes a decrease in aPKC protein expression, followed by loss of the normal stem cells at the bottom of the crypt. Without those stem cells, the crypt cells can’t regenerate. To survive, the structure can spawn either a replacement population of regenerative stem cells at the bottom, or more fetal-like stem cells at the top. These replacement cells may then lead to cancer.
“If we can better understand how aPKC protein expression is regulated, we could control and prevent tumor development, and also better understand the progression of tumors,” said Dr. Diaz-Meco. The team is now looking at aPKC expression patterns in human tumors at different stages, with hopes of developing molecular tests that could be used to detect tumors earlier, classify tumors in patients and develop better treatments.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, please see profiles for Dr. Jorge Moscat and Dr. Maria Diaz-Meco.
This research was supported in part by the National Cancer Institute of the National Institutes of Health under awards numbers, R01CA265892, R01CA250025, R01CA275846, R01CA246765, R50CA265332 and R50CA283476.
Journal
Developmental Cell