Novel radiotracer rapidly detects gastrointestinal cancer biomarker, identifies patients for targeted therapy
Reston, VA—A new PET radiotracer enables same-day imaging of the important gastrointestinal cancer biomarker known as Claudin18.2 (CLDN18.2). Uptake of the radiotracer, 68Ga-NC-BCH, was found to correlate significantly with CLDN18.2 expression, a promising finding that could allow oncologists to optimize treatment for patients and monitor their response. This research was published in the June issue of The Journal of Nuclear Medicine.
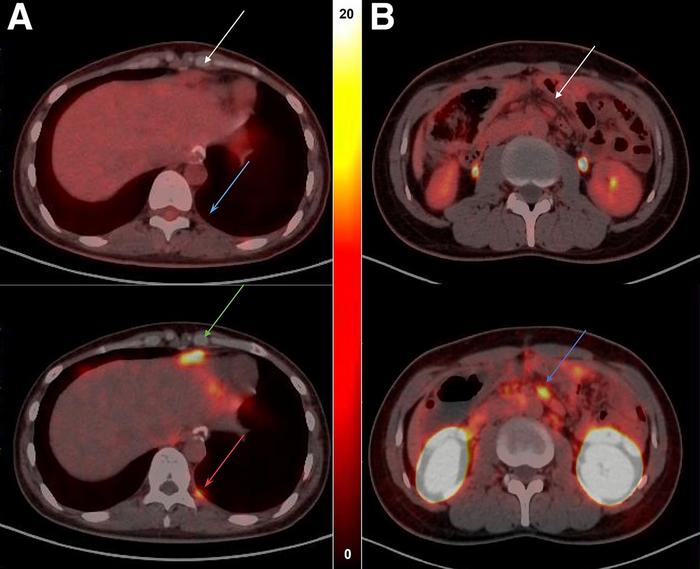
Credit: Image created by Zhi Yang, PhD, Professor at Nuclear Medicine Department, Peking University Cancer Hospital, PR China.
Reston, VA—A new PET radiotracer enables same-day imaging of the important gastrointestinal cancer biomarker known as Claudin18.2 (CLDN18.2). Uptake of the radiotracer, 68Ga-NC-BCH, was found to correlate significantly with CLDN18.2 expression, a promising finding that could allow oncologists to optimize treatment for patients and monitor their response. This research was published in the June issue of The Journal of Nuclear Medicine.
Gastrointestinal cancers are one of the most common types of cancer across the globe, accounting for more than a quarter of the total cancer incidence and more than one-third of cancer-related deaths each year. Early symptoms can be deceptive, and most gastrointestinal cancers are diagnosed at an advanced stage often leading to a poor prognosis and increased mortality.
The protein CLDN18.2 is highly expressed in gastrointestinal cancers and several forms of CDLN18.2-targeted therapies are currently undergoing clinical trials. There is no standard test for CLDN18.2, however, and most detection methods involve immunohistochemistry, an invasive process that covers only a small amount of tissue and does not reflect the heterogeneity of CLDN18.2 expression in tumors.
“The detection of CLDN18.2 expression levels is essential for identifying patients who can benefit from targeted therapies,” said Hua Zhu, PhD, professor at Peking University Cancer Hospital in Beijing, China. “In this study, we developed a CLDN18.2-targeting radiotracer and conducted whole-body PET imaging to determine its ability to detect the biomarker.”
Researchers first synthesized the radiotracer 68Ga-NC-BCH and performed preclinical evaluations on human gastrointestinal cancer cell lines and mouse models. Next, 11 patients underwent whole-body 68Ga-NC-BCH PET and 18F-FDG PET, and radiopharmaceutical biodistribution, radiation dosimetry, and the relationship between uptake and CLDN18.2 were evaluated.
68Ga-NC-BCH was stably prepared and demonstrated good radiochemical properties. The radiotracer exhibited rapid blood clearance, high affinity for CLDN18.2, and high specific uptake in CLDN18.2-positive cells and xenograft mouse models. In patients, 68Ga-NC-BCH displayed high uptake in the stomach and kidney and slight uptake in the pancreas. Compared with 18F-FDG, 68Ga-NC-BCH identified more lesions in the lymph nodes and peritoneum, the most common metastatic sites of advanced gastric cancer.
“68Ga-NC-BCH PET is a safe, noninvasive imaging method for detecting CLDN18.2 expression in patients,” said Zhu. “The rapid uptake of the radiotracer allows patients to complete the whole imaging workflow within one day, greatly increasing compliance and reducing radiation exposure. This can greatly help oncologists in making treatment decisions.”
This study was published online in April 2024.
The authors of “68Ga-NC-BCH Whole-Body PET Imaging Rapidly Targets Claudin18.2 in Lesions in Gastrointestinal Cancer Patients” include Changsong Qi, Chang Li, Miao Zhang, Cheng Zhang, Xiaotian Zhang, and Lin Shen, Department of Early Drug Development, State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers, Beijing Key Laboratory of Carcinogenesis and Translational Research, Peking University Cancer Hospital and Institute, Beijing, China; Rui Guo, Yan Chen, Xingguo Hou, Zhi Yang, and Hua Zhu, Department of Nuclear Medicine, NMPA Key Laboratory for Research and Evaluation of Radiopharmaceuticals (National Medical Products Administration), State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers, Beijing Key Laboratory of Carcinogenesis and Translational Research, Peking University Cancer Hospital and Institute, Beijing, China; Chenzhen Li and Bing Jia, Medical Isotopes Research Center, Department of Radiation Medicine, School of Basic Medical Sciences, Peking University, Beijing, China; and Bo Chen, Chengdu AlpVHHs Co. Ltd., Chengdou, China.
Visit the JNM website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed or follow us on LinkedIn.
###
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected].
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed more than 16 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit www.snmmi.org.
Journal
Journal of Nuclear Medicine
DOI
10.2967/jnumed.123.267110
Article Title
Open Access 68Ga-NC-BCH Whole-Body PET Imaging Rapidly Targets Claudin18.2 in Lesions in Gastrointestinal Cancer Patients
Article Publication Date
1-Jun-2024